Developing a rapid screening test for CoViD-19
Like the rest of the world, Australia has been hit hard by the CoViD-19 pandemic. Among the affected are institutions, such as The Australian National University (ANU). All teaching was moved online and delivered remotely, the university was swiftly closed on March 30 to help contain the spread of the virus, and research was halted suddenly. The closure impacted everyone, especially our research scientists. Most of our plant material had to be discarded, which was another setback on years of research, following the hailstorm damage on our glasshouses earlier in 2020. The transition to ‘working from home’ also had its difficulties, given lab-based research is not particularly a ‘work from home’ gig.

As we watched the case numbers for CoViD-19 seemingly sky-rocket and realised the importance of sufficient community testing, a team of scientists at the ANU, including several RIPE researchers (Sarah Rottet, Suyan Yee, and Wil Hee), volunteered to contribute our molecular biology expertise and knowledge to fighting the pandemic. Using our free time during the shutdown, we worked together, while still social distancing, to develop several diagnostic tools to easily identify CoViD-19 cases, as well as address several of the supply shortage problems the world was facing, in terms of testing kits and nasal swabs.

One such diagnostic test, which was developed within the first two months of lockdown volunteer work, included a rapid SYBR-Green-based one step qPCR assay to detect SARS-CoV-2 from saliva. This method omits the labour-intensive RNA extraction step, and combines the reverse transcription and qPCR steps into a one-step, one-pot reaction, with the aim of making it a high-throughput screening test. The use of a SYBR-Green-based technique instead of a hydrolysis probe-based qPCR also allows for the investigation of primer specificity and amplification efficiency. Through our research, we found a set of primers not currently used in any existing diagnostic kit that would be an excellent candidate for specifically screening against CoViD-19. Collecting and testing saliva also means that the discomfort associated with and the training required to administering a nasopharyngeal swab can be avoided.
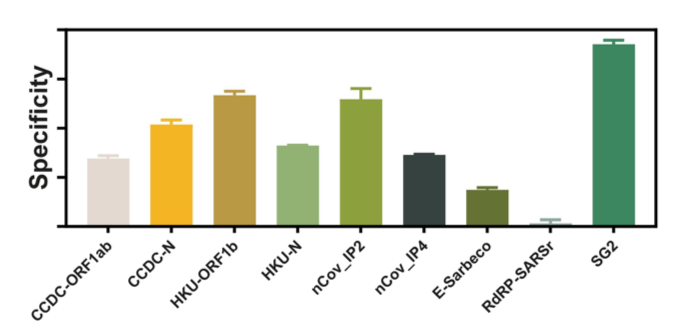
The plan was to develop a CoViD-19 screening test that is cheaper, more accurate, easier to use, scalable, and comfortable. Ultimately, our new assay reduces costs down to $4 per sample. However, the caveat is that due to the low number of active cases in Canberra, our diagnostic assay has not been vetted against infected patient saliva samples, but only non-infected samples spiked with naked SARS-CoV-2 viral RNA. As such, we ultimately recommend this SYBR-Green qPCR method as a complementary and confirmatory test used alongside existing diagnostic kits for screening for CoViD-19 cases. Our research can be found at this link on bioRxiv: https://doi.org/10.1101/2020.05.29.109702.
As we write this, phased reopening for the ANU has started, restrictions on gatherings are slowly being lifted, and life is gradually returning to a new definition of ‘normal’.
By: Suyan Yee, Wei (Wil) Hee, and Sarah Rottet || The Australian National University
