Scientists take a step closer to heat-tolerant wheat
LANCASTER, England — Researchers working on molecular-level responses in crops have taken a step closer to their goal of producing heat-tolerant wheat.
Smart thermostats tell air conditioners to switch on when the sun is bearing down in the summer and when to shut down to conserve energy. Similarly, plants have Rubisco activase, or Rca for short, that tells the plant’s energy-producing enzyme (Rubisco) to kick on when the sun is shining and signals it to stop when the leaf is deprived of light to conserve energy.
Today, a team from Lancaster University reports in The Plant Journal that swapping just one molecular building block out of 380 that make up an Rca in wheat enables it to activate Rubisco faster in hotter temperatures, suggesting an opportunity to help protect crops from rising temperatures.

“We took a wheat Rca (2β) that was already pretty good at activating Rubisco in lower temperatures and swapped out just one of its amino acids with one found in another wheat Rca (1β) that works pretty well in higher temperatures but is rubbish at activating Rubisco — and the result is a new form of 2β Rca that is the best of both worlds,” said Elizabete Carmo-Silva, a senior lecturer at the Lancaster Environment Centre who oversaw this work for a research project called Realizing Increased Photosynthetic Efficiency (RIPE). (Check out a profile on Carmo-Silva.)
RIPE is engineering crops to be more productive by improving photosynthesis, the natural process all plants use to convert sunlight into energy and yields. RIPE is supported by the Bill & Melinda Gates Foundation, the U.S. Foundation for Food and Agriculture Research (FFAR), and the U.K. Government’s Department for International Development (DFID).
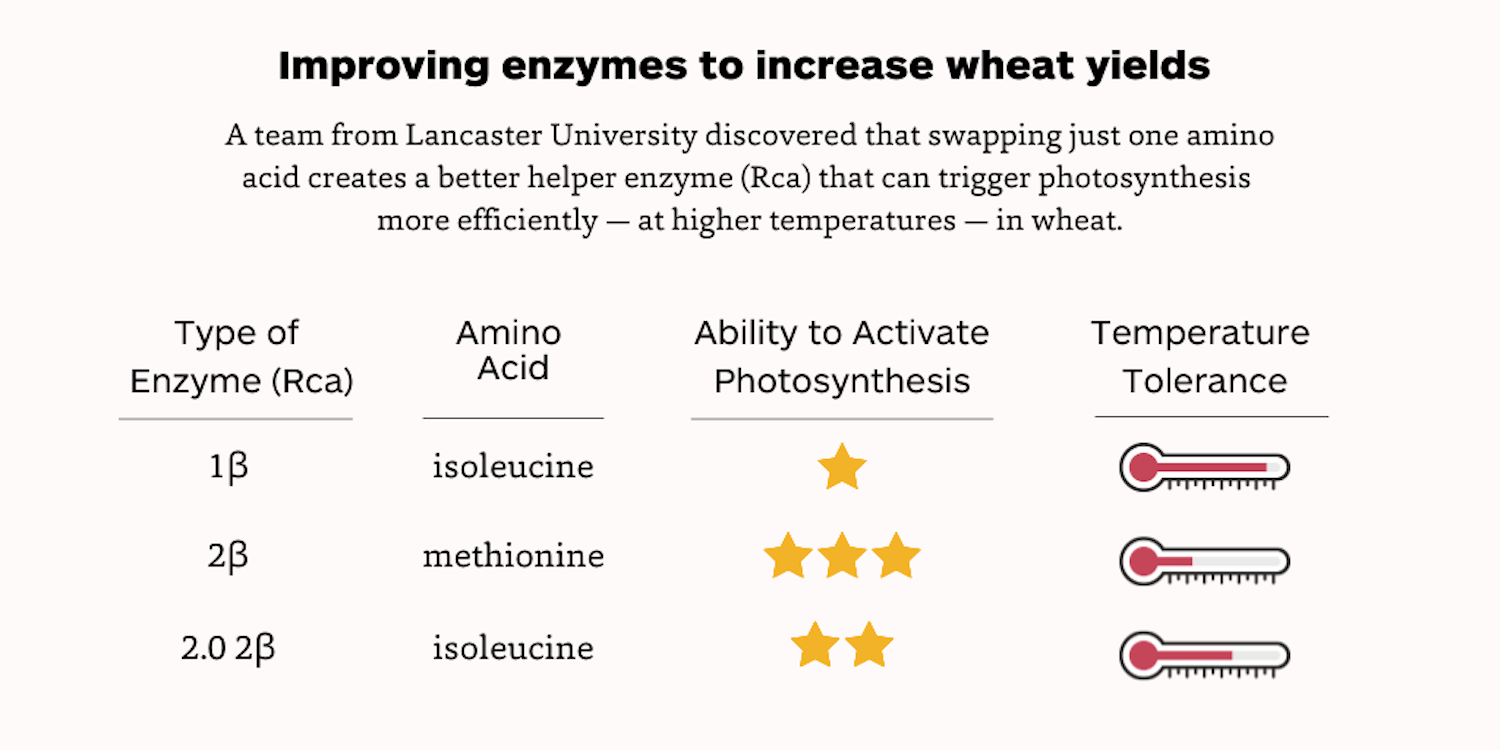
Here’s the breakdown: naturally occurring wheat Rca 1β has an isoleucine amino acid, works up to 39 degrees Celcius, but isn’t great at activating Rubisco, whereas the naturally occurring 2β has a methionine amino acid, works up to about 30 degrees Celcius, and is good at activating Rubisco. Here the team has created a new version of 2β with an isoleucine amino acid that works up to 35 degrees Celcius and is quite good at activating Rubisco.
"Essentially, 1β is a rubbish enzyme and 2β is sensitive to higher temperatures,” Carmo-Silva said. “The cool thing here is that we have shown how this one amino acid swap can make Rca active at higher temperatures without really affecting its efficiency to activate Rubisco, which could help crops kickstart photosynthesis under temperature stress to churn out higher yields.”
This work was carried out in vitro in E. coli, supported by a Ph.D. studentship by the Lancaster Environment Centre to first author Gustaf Degen. Importantly, these findings will support RIPE’s efforts to characterize and improve the Rca of other food crops such as cowpea and soybean, each with multiple different forms of Rca.
“When looking at cowpea growing regions in Africa, it goes all the way from South Africa with an average around 22 degrees Celcius to Nigeria at about 30, and areas further north get to 38,” Carmo-Silva said. “If we can help Rubisco activate more efficiently across these temperatures, that is really powerful and could help us close the gap between yield potential and the reality for farmers who depend on these crops for their sustenance and livelihoods.”
The RIPE project and its sponsors are committed to ensuring Global Access and making the project’s technologies available to the farmers who need them the most.
Realizing Increased Photosynthetic Efficiency (RIPE) aims to improve photosynthesis to equip farmers worldwide with higher-yielding crops to ensure everyone has enough food to lead a healthy, productive life. This international research project is sponsored by the Bill & Melinda Gates Foundation, the U.S. Foundation for Food and Agriculture Research (FFAR), and the U.K. Government's Department for International Development (DFID).
RIPE is led by the University of Illinois in partnership with The Australian National University, Chinese Academy of Sciences, Commonwealth Scientific and Industrial Research Organisation, Lancaster University, Louisiana State University, University of California, Berkeley, University of Cambridge, University of Essex, and U.S. Department of Agriculture, Agricultural Research Service.
